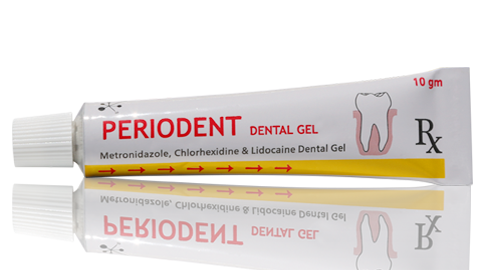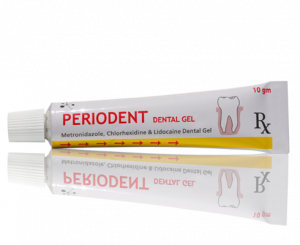
Periodent Dental Gel
INDICATION:
Gingivitis, teething pain, irritation, cold sores & aphthous-ulceration. Oral candidal or bacterial infections also. It is indicated to reduce dental plague accumulation. It is indicated for patients having coexisting gingivitis and periodontis.
FORMULATION:
Metronidazole 1.0% w/w
(As Metronidazole Benzoate)
Chlorhexidine Gluconate Solution 0.25% w/w
Lidocaine Hydrochloride 2.0 w/w
(equivalent to Anhydrous Lidocaine Hydrochloride)
Water Soluble Gel Base q.s.
PHARMACEUTICAL DOSAGE FORM:
Dental Gel
PRECAUTIONS:
“Carefully read the instructions before use. Consult your doctor for further information.”
“Use upon doctor’s prescription only”
NECESSARY INFORMATION BEFORE USE:
Contraindications:
It should not be used in any patient who has a known sensitivity to Chlorhexidine, Metronidazole and Lidocaine Hydrochloride or any of the ingredients of the product.
Precautions:
For patients having coexisting gingivitis and periodontitis, the presence or absence of gingival inflamation following treatment with PERIODENT Dental gel should not be used as major indicator of underlying periodontis.
PERIODENT Dental gel can cause staining of oral surfaces, such as tooth surfaces, restorations, and the dorsum of the tongue. Stain will be more pronounced in patients who have heavier accumulations of un-removed plaque. Stain can be removed from most tooth surfaces by a conventional professional prophylactic technique. If natural stain can not be removed form these surfaces be dental prophylaxis, patients should be excluded from PERIODENT Dental gel treatment if permanent discoloration is unacceptable.
Patients information
For oropharyngal use only. Avoid contact with eye.
Patients should be instructed to strictly adhere to dosage instructions and to keep the medication out of the reachof children.
Drug Interactions
No reports of drug interaction were located.
ADHERE EFFECTS:
The most common side effects are an increase staining of teeth and other oral surfaces, an increase in calculus formation adn an alteration in taste perception. No serious systemic adverse reactions associated with use of PERIODENT dental gel were observed i clinical testing. Minor irritation and superficial desquamation of the oral mucosa have been noted in patients using PERIODENT dental gel, particularly among children. “Inform your doctor in case of any adverse reactions related to drug use”
INSTRUCTIONS FOR USE:
Dosage ad Administration
Adult: Apply enough gel to cover the finger tip and rub gently onto the effected area, not more often than 3 hourly.
Children: Not recommended for children below 4 months.
SHELF LIFE:
24 months from the date of manufacturing
STORAGE:
Storage at the temperature not exceeding 30 degree celcius.
Protect from light. Keep out of the reach of children.
PRESENTATION:
10 gram gel in an Aluminum collapsible tube.
20 gram gel in an Aluminum collapsible tube.
Each tube is packed in an unit carton with package insert.